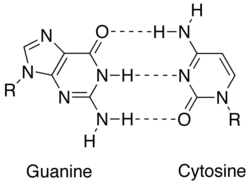
Match up between 2 DNA bases (guanine and cytosine) showing hydrogen bonds (dashed lines) holding them together

Mate up between two DNA bases (adenine and thymine) showing hydrogen bonds (broken lines) holding them together
In unit biology, complementarity describes a relationship between two structures apiece following the lock-and-key out precept. In nature complementarity is the base principle of DNA replica and transcription as it is a belongings shared between deuce DNA or RNA sequences, much that when they are aligned antiparallel to each other, the nucleotide bases at each position in the sequences will be complementary color, much like looking in the mirror and sightedness the reverse of things. This complementary alkali pairing allows cells to copy information from one generation to another and even find and reanimate damage to the information stored in the sequences.
The degree of complementarity between two nucleic acid strands may vary, from thoroughgoing complementarity (each nucleotide is across from its opposite) to no complementarity (each nucleotide is not across from its opposite) and determines the constancy of the sequences to be together. What is more, various DNA furbish up functions as asymptomatic as regulatory functions are supported ignoble pair complementarity. In biotechnology, the principle of base pair complementarity allows the generation of DNA hybrids between RNA and DNA, and opens the room access to innovative tools such as cDNA libraries. While most complementarity is seen between two separate strings of DNA or Ribonucleic acid, it is too possible for a sequence to own internal complementarity sequent in the sequence binding to itself in a folded configuration.
DNA and RNA base span complementarity [cut]

Complementarity between two antiparallel strands of DNA. The top strand goes from the left to the right and the bring dow strand goes from the right to the left lining them upwardly.

Leftist: the nucleotide base pairs that hindquarters form in double-isolated DNA. Betwixt A and T there are ii atomic number 1 bonds, while there are three between C and G. The right way: two complementary strands of DNA.
Complementarity is achieved by distinct interactions between nucleobases: adenine, thymine (uracil in RNA), guanine and C. A and guanine are purines, while thymine, cytosine and uracil are pyrimidines. Purines are larger than pyrimidines. Both types of molecules complement from each one other and dismiss only base pair with the opposing type of nucleobase. In nucleic acid, nucleobases are held in concert aside hydrogen bonding, which merely works expeditiously between adenine and thymine and between guanine and cytosine. The base complement A = T shares two hydrogen bonds, while the base pair G ≡ C has three hydrogen bonds. All otherwise configurations between nucleobases would hinder double helix organisation. DNA strands are destined in opposite directions, they are said to be antiparallel.[1]
| Nucleic Acid | Nucleobases | Base complement |
| DNA | adenine(A), T(T), G(G), C(C) | A = T, G ≡ C |
| RNA | adenine(A), uracil(U), guanine(G), cytosine(C) | A = U, G ≡ C |
A complementary strand of DNA or Ribonucleic acid may be constructed supported nucleobase complementarity.[2] Each Base pair, A = T vs. G ≡ C, takes up roughly the same space, thereby sanctionative a twisted DNA double helix formation without some spatial distortions. H bonding between the nucleobases also stabilizes the DNA double helix.[3]
Complementarity of DNA strands in a two-base hit helix attain it possible to apply one strand as a template to fabricate the otherwise. This principle plays an important role in DNA retort, setting the foundation of heredity by explaining how beginning information butt be passed down to the next generation. Complementarity is also utilized in DNA transcription, which generates an RNA strand from a DNA template.[4] In addition, HIV, a fiber RNA virus, encodes an RNA-parasitic DNA polymerase (reverse transcriptase) that uses complementarity to catalyze genome replication. The reverse transcriptase can switch between two parental Ribonucleic acid genomes by copy-choice recombination during return.[5]
DNA restore mechanisms such as proof reading material are complementarity based and reserve for error correction during DNA replication by removing mismatched nucleobases.[1] In general, damages in unrivalled strand of DNA terminate be repaired by removal of the damaged section and its replacement by victimisation complementarity to copy data from the other strand, As occurs in the processes of mismatch repair, nucleotide deletion repair and base excision repair.[6]
Nucleic acids strands may also form hybrids in which single stranded DNA may readily anneal with complementary DNA or RNA. This precept is the basis of commonly performed laboratory techniques such as the polymerase strand reaction, PCR.[1]
Two strands of complemental sequence are referred to as common sense and opposed-gumption. The sense Strand is, generally, the transcribed sequence of DNA or the Ribonucleic acid that was generated in transcription, while the anti-sense strand is the filament that is complementary to the sense sequence.
Self-complementarity and hairpin loops [delete]

A sequence of RNA that has internal complementarity which results in it folding into a hairpin
Self-complementarity refers to the fact that a sequence of DNA Oregon RNA may sheep pen back on itself, creating a double-strand like structure. Depending on how surrounding together the parts of the successiveness are that are self-complementary, the fibril may form hairpin loops, junctions, bulges or intimate loops.[1] RNA is more in all likelihood to mannequin these kinds of structures callable to base pair binding not seen in Desoxyribonucleic acid, such every bit G book binding with uracil.[1]

A sequence of RNA showing hairpins (far right and far upper left-of-center), and internal loops (lour left structure)
Regulatory functions [edit out]
Complementarity can be found between short nucleic acid stretches and a coding region or an transcribed cistron, and results in base pairing. These short nucleic acid sequences are commonly found in nature and hold regulatory functions such equally factor silencing.[1]
Antisense transcripts [edit]
Antisense transcripts are stretches of non coding mRNA that are complementary to the secret writing sequence.[7] Genome all-encompassing studies give shown that RNA antisense transcripts occur commonly within nature. They are generally believed to increase the coding possible of the genetic write in code and add an overall layer of complexity to gene regulation. So removed, it is well-known that 40% of the human genome is canned in some directions, underlining the potential significance of reverse recording.[8] Information technology has been suggested that complementary regions between sentiency and antisense transcripts would let generation of double stranded RNA hybrids, which Crataegus laevigata play an important role in factor regulation. For example, hypoxia-induced factor 1α mRNA and β-secretase mRNA are transcribed bidirectionally, and it has been shown that the antisense copy acts A a stabilizer to the sense script.[9]
miRNAs and siRNAs [edit]

Formation and function of miRNAs in a cell
miRNAs, microRNA, are short RNA sequences that are additive to regions of a transcribed gene and have regulatory functions. Current research indicates that circulating miRNA Crataegus laevigata be utilized as refreshing biomarkers, hence register promising bear witness to be utilized in disease diagnostics.[10] MiRNAs are formed from thirster sequences of RNA that are cut free by a Dicer enzyme from an RNA sequence that is from a regulator factor. These short strands bind to a RISC complex. They match up with sequences in the upriver region of a transcribed gene due to their complementarity to act as a silencer for the gene in trine ways. One is by preventing a ribosome from binding and initiating translation. Two is by degrading the informational RNA that the complex has bound to. And threesome is by providing a new double-stranded RNA (dsRNA) chronological sequence that Dicer rear human activity upon to create more miRNA to find and degrade more copies of the gene. Small interfering RNAs (siRNAs) are corresponding in function to miRNAs; they come from separate sources of RNA, but serve a confusable purpose to miRNAs.[1] Given their improvident length, the rules for complementarity means that they can still be very discriminating in their targets of choice. Given that there are four choices for each base in the strand and a 20bp - 22bp length for a mi/siRNA, that leads to more than 1×1012 imaginable combinations. Given that the human genome is ~3.1 jillio bases in length,[11] this substance that each miRNA should only find a match once in the intact human genome by fortuity.
Kissing hairpins [edit]
Hugging hairpins are formed when a single strand of nucleic acid complements with itself creating loops of RNA in the form of a hairpin.[12] When two hairpins inherit contact with from each one other in vivo, the complementary bases of the two strands descriptor up and begin to unwind the hairpins until a dual-stranded Ribonucleic acid (dsRNA) convoluted is formed or the complex unwinds rearward to two separate strands due to mismatches in the hairpins. The secondary structure of the hairpin prior to cuddling allows for a stable structure with a relatively fixed interchange in energy.[13] The role of these structures is a reconciliation of stability of the hairpin loop vs binding strength with a complementary strand. Too strong an initial binding to a bad emplacemen and the strands will not unwind quickly enough; too weak an initial binding and the strands will ne'er fully form the desirable complex. These hairpin structures let for the pic of enough bases to provide a strong enough check happening the initial binding and a weak enough internal valid to allow the unfolding once a indulgent pit has been found.[13]
---C G--- C G ---C G--- U A C G G C U A C G G C A G C G A A A G C U A A U CUU ---CCUGCAACUUAGGCAGG--- A GAA ---GGACGUUGAAUCCGUCC--- G A U U U U U C U C G C G C C G C G A U A U G C G C ---G C--- ---G C--- Hugging hairpins encounter up at the top off of the loops. The complementarity of the two heads encourages the hairpin to open and straighten out to become one flat sequence of deuce strands rather than 2 hairpins.
Bioinformatics [edit out]
Complementarity allows information found in DNA or RNA to be stored in a single strand. The complementing chain can be observed from the templet and contrariwise as in cDNA libraries. This also allows for psychoanalysis, the likes of comparing the sequences of two different species. Shorthands have been industrial for penning down sequences when on that point are mismatches (ambiguity codes) or to speed up how to read the opposite sequence in the complement (ambigrams).
cDNA Library [blue-pencil]
A cDNA library is a collection of expressed DNA genes that are seen as a useful quotation tool in gene identification and cloning processes. cDNA libraries are constructed from mRNA victimization RNA-dependent DNA polymerase reverse transcriptase (RT), which transcribes an messenger RNA templet into DNA. Consequently, a cDNA library can only hold back inserts that are meant to Be transcribed into mRNA. This process relies on the principle of DNA/RNA complementarity. The end product of the libraries is double stranded DNA, which English hawthorn be inserted into plasmids. Hence, cDNA libraries are a powerful tool in modern explore.[1] [14]
Ambiguity codes [edit]
When writing sequences for tabular biology information technology may be necessary to have IUPAC codes that mean "any of the 2" or "any of the three". The IUPAC code R (whatsoever purine) is complementary to Y (any pyrimidine) and M (amino) to K (keto). W (weak) and S (strong) are usually not swapped[15] but hold been swapped in the past past some tools.[16] W and S denote "weak" and "strong", respectively, and indicate a number of the hydrogen bonds that a nucleotide uses to pair with its complementing pardner. A cooperator uses the same number of the bonds to make a complementing pair.[17]
An IUPAC code that specifically excludes one of the trey nucleotides hindquarters be complementary to an IUPAC code that excludes the complementary nucleotide. For exemplify, V (A, C or G - "not T") stool be complementary to B (C, G or T - "not A").
| Symbol[18] | Verbal description | Bases represented | ||||
|---|---|---|---|---|---|---|
| A | adenine | A | 1 | |||
| C | cytosine | C | ||||
| G | guanine | G | ||||
| T | thymine | T | ||||
| U | uracil | U | ||||
| W | weak | A | T | 2 | ||
| S | strong | C | G | |||
| M | amino | A | C | |||
| K | keto | G | T | |||
| R | Purine | A | G | |||
| Y | pyrimidine | C | T | |||
| B | non A (B comes after A) | C | G | T | 3 | |
| D | not C (D comes after C) | A | G | T | ||
| H | non G (H comes after G) | A | C | T | ||
| V | not T (V comes subsequently T and U) | A | C | G | ||
| N or - | any base (not a gap) | A | C | G | T | 4 |
Ambigrams [edit]
Specific characters Crataegus oxycantha be in use to create a suitable (ambigraphic) nucleic Zen notation for complementary bases (i.e. G = b, cytosine = q, adenine = n, and thymine = u), which makes IT is viable to full complement entire DNA sequences by bu rotating the text "upside pour down".[19] For illustrate, with the previous alphabet, buqn (GTCA) would read every bit ubnq (TGAC, reverse full complement) if turned upside down.
- qqubqnnquunbbqnbb
- bbnqbuubnnuqqbuqq
Ambigraphic notations readily visualize complementary nucleic acid stretches such as palindromic sequences.[20] This feature is enhanced when utilizing custom fonts operating theatre symbols rather than ordinary ASCII or even Unicode characters.[20]
Get a line besides [edit]
- Base pair
References [edit]
- ^ a b c d e f g h Watson, James, Frigid Spring Haven Laboratory, Tania A. Baker, MIT, Stephen P. Bell, Massachusetts Institute of Technology, Alexander Gann, Cold Take shape Harbor Research lab, Michael Levine, University of California, Bishop Berkeley, Richard Losik, Harvard University ; with Stephen C. Harrison, Harvard Medical (2014). Molecular biology of the gene (Seventh male erecticle dysfunction.). Hub of the Universe: Benjamin-E. e. cummings Publishing firm. ISBN978-0-32176243-6.
- ^ Pray, Leslie (2008). "Uncovering of DNA structure and function: Watson and Crick". Nature Education. 1 (1): 100. Retrieved 27 November 2013.
- ^ Shankar, A; Jagota, A; Mittal, J (Oct 11, 2012). "DNA base dimers are stabilized by hydrogen-bonding interactions including non-Watson-Crick mating draw near graphite surfaces". The Daybook of Physical Chemistry B. 116 (40): 12088–94. Interior:10.1021/jp304260t. PMID 22967176.
- ^ Hood, L; Galas, D (Jan 23, 2003). "The integer code of DNA". Nature. 421 (6921): 444–8. Bibcode:2003Natur.421..444H. doi:10.1038/nature01410. PMID 12540920.
- ^ Rawson JMO, Nikolaitchik OA, Keele BF, Pathak VK, Hu WS. Recombination is required for efficient Human immunodeficiency virus-1 replication and the maintenance of viral genome integrity. Nucleic Acids Res. 2018;46(20):10535-10545. DOI:10.1093/nar/gky910 PMID 30307534
- ^ Fleck O, Nielsen O. DNA reparation. J Cell Sci. 2004;117(Atomic number 78 4):515-517. DOI:10.1242/jcs.00952
- ^ Atomic number 2, Y; Vogelstein, B; Velculescu, VE; Papadopoulos, N; Kinzler, KW (Dec 19, 2008). "The antisense transcriptomes of human cells". Science. 322 (5909): 1855–7. Bibcode:2008Sci...322.1855H. doi:10.1126/science.1163853. PMC2824178. PMID 19056939.
- ^ Katayama, S; Tomaru, Y; Kasukawa, T; Waki, K; Nakanishi, M; Nakamura, M; Nishida, H; Yap, Cubic centimetre; Suzuki, M; Kawai, J; Suzuki, H; Carninci, P; Hayashizaki, Y; Wells, C; Frith, M; Ravasi, T; Pang, KC; Hallinan, J; Mattick, J; Hume, DA; Lipovich, L; Batalov, S; Engström, PG; Mizuno, Y; Faghihi, MA; Sandelin, A; Chalk, AM; Mottagui-Tabar, S; Liang, Z; Lenhard, B; Wahlestedt, C; RIKEN Genome Exploration Research Group; Genome Science Chemical group (Genome Network Project Core Group); FANTOM Consortium (Sep 2, 2005). "Antisense transcription in the mammalian transcriptome". Science. 309 (5740): 1564–6. Bibcode:2005Sci...309.1564R. doi:10.1126/science.1112009. PMID 16141073. S2CID 34559885.
- ^ Faghihi, MA; Zhang, M; Huang, J; Modarresi, F; New wave der Brug, Military police; Nalls, MA; Cookson, MR; St-Laurent G, 3rd; Wahlestedt, C (2010). "Evidence for natural antisense transcript-mediated inhibition of microRNA function". Genome Biological science. 11 (5): R56. doi:10.1186/gilbert-2010-11-5-r56. PMC2898074. PMID 20507594.
- ^ Kosaka, N; Yoshioka, Y; Hagiwara, K; Tominaga, N; Katsuda, T; Ochiya, T (September 5, 2013). "Trash or Treasure: animate thing microRNAs and cell-to-cell communication". Frontiers in Genetics. 4: 173. doi:10.3389/fgene.2013.00173. PMC3763217. PMID 24046777.
- ^ "Ensembl genome browser 73: Homo sapiens - Gathering and Genebuild". Ensembl.org . Retrieved 27 November 2013.
- ^ Marino, JP; Gregorian RS, Jr; Csankovszki, G; Crothers, DM (Jun 9, 1995). "Bent helix formation betwixt RNA hairpins with complemental loops". Science. 268 (5216): 1448–54. Bibcode:1995Sci...268.1448M. doi:10.1126/skill.7539549. PMID 7539549.
- ^ a b Chang, KY; Tinoco I, Jr (Whitethorn 30, 1997). "The structure of an RNA "kissing" hairpin multiplex of the HIV TAR hairpin loop and its full complement". Journal of Unit Biology. 269 (1): 52–66. doi:10.1006/jmbi.1997.1021. PMID 9193000.
- ^ Wan, KH; Yu, C; George, RA; Carlson, JW; Hoskins, Right ascension; Svirskas, R; Stapleton, M; Celniker, SE (2006). "Utmost-throughput plasmid cDNA library screening". Nature Protocols. 1 (2): 624–32. doi:10.1038/nprot.2006.90. PMID 17406289. S2CID 205463694.
- ^ Jeremiah Faith (2011), conversion table
- ^ arep.Master of Education.John Harvard.edu A tool page with the note astir the applied W-S transition patch.
- ^ Reverse-full complement tool page with documented IUPAC code transition, source code available.
- ^ Nomenclature Committee of the International Union of Biochemistry (Tar Heel State-IUB) (1984). "Nomenclature for Incompletely Specific Bases in Nucleic Acid Sequences". Retrieved 2008-02-04 .
- ^ Rozak DA (2006). "The practical and pedagogic advantages of an ambigraphic nucleic acid notation". Nucleosides Nucleotides Nucleic Acids. 25 (7): 807–13. Interior Department:10.1080/15257770600726109. PMID 16898419. S2CID 23600737.
- ^ a b Rozak, DA; Rozak, AJ (May 2008). "Simplicity, function, and discernability in an enhanced ambigraphic nucleic acid notation". BioTechniques. 44 (6): 811–3. doi:10.2144/000112727. PMID 18476835.
External golf links [edit]
- Reverse complement tool
- Reverse Complement Puppet @ DNA.UTAH.EDU
write the base sequence of the complementary dna strand
Source: https://en.wikipedia.org/wiki/Complementarity_(molecular_biology)